- Research
- Open access
- Published:
Machine learning for prediction of asthma exacerbations among asthmatic patients: a systematic review and meta-analysis
BMC Pulmonary Medicine volume 23, Article number: 278 (2023)
Abstract
Background
Asthma exacerbations reduce the patient’s quality of life and are also responsible for significant disease burdens and economic costs. Machine learning (ML)-based prediction models have been increasingly developed to predict asthma exacerbations in recent years. This systematic review and meta-analysis aimed to identify the prediction performance of ML-based prediction models for asthma exacerbations and address the uncertainty of whether modern ML methods could become an alternative option to predict asthma exacerbations.
Methods
PubMed, Cochrane Library, EMBASE, and Web of Science were searched for studies published up to December 15, 2022. Studies that applied ML methods to develop prediction models for asthma exacerbations among asthmatic patients older than five years and were published in English were eligible. The prediction model risk of bias assessment tool (PROBAST) was utilized to estimate the risk of bias and the applicability of included studies. Stata software (version 15.0) was used for the random effects meta-analysis of performance measures. Subgroup analyses stratified by ML methods, sample size, age groups, and outcome definitions were conducted.
Results
Eleven studies, including 23 prediction models, were identified. Most of the studies were published in recent three years. Logistic regression, boosting, and random forest were the most used ML methods. The most common important predictors were systemic steroid use, short-acting beta2-agonists, emergency department visit, age, and exacerbation history. The overall pooled area under the curve of the receiver operating characteristics (AUROC) of 11 studies (23 prediction models) was 0.80 (95% CI 0.77–0.83). Subgroup analysis based on different ML models showed that boosting method achieved the best performance, with an overall pooled AUROC of 0.84 (95% CI 0.81–0.87).
Conclusion
This study identified that ML was the potential tool to achieve great performance in predicting asthma exacerbations. However, the methodology within these models was heterogeneous. Future studies should focus on improving the generalization ability and practicability, thus driving the application of these models in clinical practice.
Background
Asthma is a chronic heterogeneous disease affecting approximately 241 million people worldwide [1]. Despite many effective medicines available, a proportion of asthmatic patients have uncontrolled asthma and asthma exacerbations [2, 3]. Asthma exacerbations are characterized by progressive deterioration of asthma-related symptoms and lung function, resulting in a poor quality of life [4, 5]. Severe asthma exacerbations are also responsible for decreased lung function, hospitalization, and even death, thus leading to disease and economic burdens [6, 7]. Early recognition and timely intervention are the best strategies to prevent severe asthma exacerbations. Therefore, identifying patients at high risk of asthma exacerbations is crucial.
According to a systematic review including ten prediction models for asthma exacerbations, the best prediction performance was achieved by logistic regression (LR) with a c-statistic of 0.80 [8]. However, this systematic review did not include models based on modern machine learning (ML) algorithms, such as random forest (RF), neural network (NN), boosting algorithms, and support vector machine (SVM). ML has become a popular method for developing prediction models in the medical field due to its ability to process complex, massive health data [9]. Many studies developing prediction models for asthma exacerbations based on ML methods have been published, especially in recent years [10, 11]. However, few systematic reviews were conducted to evaluate these existing ML models. Therefore, we perform a systematic review and meta-analysis to estimate the prediction performance of ML-based prediction models for asthma exacerbations and identify whether modern ML methods could become an alternative option to prediction.
Methods
Search protocol
We conducted this systematic review in accordance with Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (The PRISMA-DTA Statement). The protocol of this systematic review was registered and published on PROSPERO (reference number CRD42022380059).
Search strategy
PubMed, Cochrane Library, EMBASE, and Web of Science were searched for relevant literature published from the earliest available online date up to December 15, 2022. Our search strategies used controlled terms and free-text terms to search for studies of the ML approach and asthma exacerbations. Details of the search strategy are given in Additional file 1. We also checked reference lists of previous systematic reviews for potentially relevant papers.
Eligibility criteria and study selection
All search records were exported from the four databases and imported to EndNote 20 (Clarivate), a reference management tool, for compiling and duplication checking. After removing the duplications, two reviewers (SQ, X and XY, J) independently screened the titles and abstracts to select the studies based on inclusion criteria. Subsequently, they screened the full texts to select eligible studies. Any discrepancies were resolved by a third reviewer (W, C).
All studies should fulfill the criteria as follows:
-
a
Studies must be published in English.
-
b
Focused on participants aged five years and older with pre-existing asthma diagnoses.
-
c
Utilized machine learning algorithms to generate prediction models.
-
d
Aimed to predict patients who would suffer asthma exacerbations in the future.
-
e
Evaluated the prediction performance of models on a validation dataset.
-
f
Provided a clear description of ML methods and input features (predictors).
-
g
Provided the performance metrics regarding sensitivity and specificity.
We did not limit the type of publication and study designs.
Data extraction
Two reviewers (SQ, X and Y, J) independently read the full texts of eligible studies and extracted data elements, including (1) the paper source, (2) study information, (3) prediction models, (4) performance measures, (5) population characteristics, and (6) outcomes. Full details of data extraction are provided in Additional file 2. We defined asthma exacerbations in accordance with an Official American Thoracic Society/European Respiratory Society (ATS/ERS) Statement [12]. Briefly, severe asthma exacerbation should include (1) at least three days of systemic corticosteroid treatment or (2) a hospitalization/emergency department visit for asthma requiring systemic corticosteroids. Moderate asthma exacerbation should include (1) at least two days of symptoms and lung function deterioration, requiring increasing bronchodilator use, or (2) visits for asthma not requiring systemic corticosteroids intervention. Using available statistics in the manuscripts, we manually calculated parameters not reported (e.g., the number of positive cases). We also emailed the corresponding author(s) for missing data.
Quality and bias assessment
There are no widely accepted tools for assessing the quality of machine learning-based research in medical fields. In 2019, Wolff et al. [13] developed the prediction model risk of bias assessment tool (PROBAST), which could assess the risk of bias (ROB) and the applicability of prediction model studies. For ROB assessment, PROBAST includes four domains: participants, predictors, outcomes, and analysis. Each domain contains 2 to 9 signaling questions that facilitate this domain’s ROB assessment (low, high, or unclear). The overall ROB assessment for a study is “low,” “high,” or “unclear,” based on each domain’s ROB classification. The first three domains with review questions are also used for applicability judgment (low, high, or unclear concern). This paper used the PROBAST to assess the ROB and applicability of included studies. Two authors (SQ, X and CH, L) independently assessed eligible studies, and any disagreements were resolved by discussion.
Data analysis
We narratively described these included studies, such as distribution of publication year, population characteristics, popular machine learning methods, validation methods, and important features. For studies that were able to calculate the number of true positive cases, true negative cases, false positive cases, and false negative cases on the validation dataset, the overall pooled area under the curve of the receiver operating characteristics (AUROC), sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were estimated using random effects meta-analysis. I2 was used to describe the percentage of the variability in effect estimates due to heterogeneity.
A small sample size causes the risk of overfitting, which may lead to poor generalization of prediction models. Subgroup analysis was stratified by sample size (< 10000 participants/ > 10000 participants). In addition, we performed a subgroup analysis of ML methods (LR, boosting, and RF), age groups (children only, children and adults, and adults only), and different outcome definitions. Univariate and multivariate random-effects meta-regression for sample size, ML methods, age groups, outcome definitions, and publication year was performed to explore the source of heterogeneity. For clarity, we referred to factors used for model development as “predictors” and the factors used for meta-regression at study level as “variables”. Sensitivity analyses were conducted to examine the robustness of the result by excluding each study. Deeks’ funnel plot was applied to test publication bias. We conducted all our analyses using Stata software (version 15.0). We used the MADIS module for pooling performance measures and the “metareg” macro for conducting the meta-regression analysis. The commands used in the analysis are provided in Additional file 2.
Results
Study selection
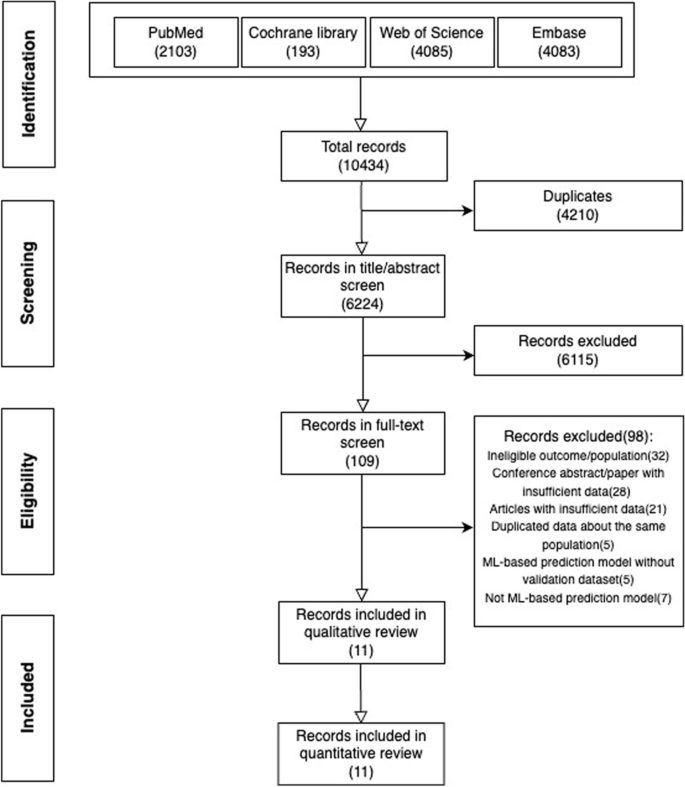
A total of 10434 papers were identified from four databases (PubMed (2013), Cochrane library (193), Web of Science (4085), Embase (4083)) (see Additional file 1). After excluding 4210 duplicates, we browsed titles and abstracts of the remaining 6224 papers resulting in 109 papers that might be eligible based on the pre-defined selection criteria. Then, we screened these papers’ full texts and supplementary materials and included 11 papers for synthesis (Fig. 1). Two studies included participants without age limitation, but only a tiny proportion of participants in these two studies were aged younger than five years old [14, 15].
Study characteristics
The publication year of these papers ranged from 1999 to 2022, and more than half of them (6/11) were published in recent three years. Ten studies were retrospective, and the remaining one was prospective. The minimum and maximum number of included participants for prediction model development were 94 and 782762, respectively. The proportion of outcome events ranged from 0.2% to 32.8% (Table 1).
Most studies (9/11) included asthmatic participants regardless of asthma severity, control levels, or treatment. Only two studies mentioned additional criteria, such as participants with mild-moderate asthma [19, 24] and stable asthma [24] (see Additional file 3). Prediction windows also varied from several days to 4 years, with seven studies setting the prediction window within one year (Table 1). For outcome events (see Additional file 3), nine studies defined asthma exacerbations as asthma-related hospitalization or emergency department visit according to the asthma-related diagnosis code [14,15,16,17,18, 21], medical records [22, 23], or questionnaires [19]. Two studies used the definitions in accordance with the ATS/ERS recommendation [20, 24].
ML algorithms and validation methods
Eleven studies developed a total of 23 ML-based prediction models. The most popular ML algorithm was LR, followed by RF, XGBoost, and LGBoost (Fig. 2a). Validation methods were used in 6 studies, such as cross-validation [15, 19, 24], bagging [20], and split-sample validation [16] (Table 1). For the generalization test, ten studies used external validation. One study split a single dataset into a training dataset and a test dataset and used the latter to assess the generalization ability of prediction models. We also included more detailed descriptions of the dataset and the validation method in Additional file 4 for better clarity.
Predictors in ML models
A wide range of predictors was used in these studies, such as demographic factors, clinical-related factors, and socioeconomic factors. Clinical-related factors (n = 11) and demographic factors (n = 7) were used most in the final models, followed by social-economic factors (n = 3) (see Additional file 5). The number of predictors in best prediction models ranged from 1 to 221. Most studies that applied LR and classification and regression trees (CART) to develop prediction models had a relatively minor number of predictors. The number of predictors in models based on boosting and RF was much higher (Table 1). All studies reported the predictors' contributions or odds ratios (only in LR). Among these important predictors, systemic steroids use, short-acting beta2-agonists, and emergency department visit were the most common predictors, followed by age, asthma diagnosis number, and exacerbation history (Fig. 2b, Additional file 5).
Risk of bias and applicability
The overall quality assessment (ROB and applicability) based on PROBAST is shown in Table 1. Additional file 6 provides judgment details of each study. The overall bias of all studies was rated as high risk. For participants, eight studies were at high ROB mainly due to retrospective design and asthma definition that was based on asthma-related medicine use and doctors’ diagnosis. The bias of predictors mainly results from subjective predictors (such as self-report symptoms), auxiliary examinations from different medical institutions, and comorbidities. These factors were difficult to be defined consistently. The definition of asthma exacerbations given by the ATS/ERS statement is widely accepted [12]. Studies in which the outcome was not in accordance with ATS/ERS statement were rated as high risk of bias. All studies had a high risk of bias in the “analysis” domain.
For applicability assessment, one study was judged as low concerns, and the remains were rated as high concerns. Two studies included asthmatic participants with mild to moderate asthma [19, 24], thus might reduce the generalizability and applicability. Six studies were assessed as having high concerns in the “predictors” domain. The applicability would reduce when predictors were challenging to be defined similarly. As for the outcome, studies (10/11) would receive a rating of high concern if they did not focus on moderate to severe asthma exacerbations defined by the ATS/ERS statement.
Meta-analysis
The discrimination ability of ML-based models was various. AUROC was reported in 21 models, the best prediction performance of asthma exacerbations ranged from 0.59 to 0.90. The specificity and sensitivity based on different cut-off points were reported in all included studies, with the range of 0.54–0.93 and 0.25–0.88, respectively. Negative predictive value (n = 4), positive predictive value (n = 4), and accuracy (n = 4) of prediction models in several studies were also reported (Table 1).
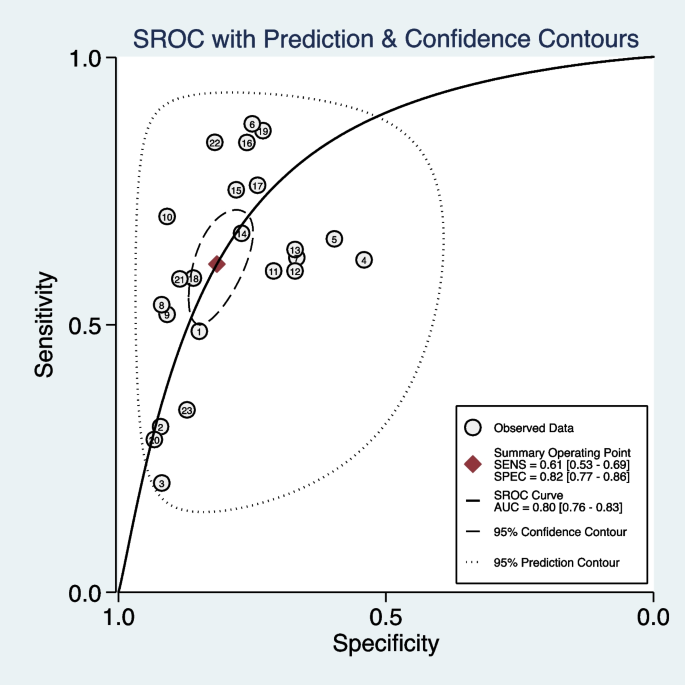
We included 11 studies (23 models) with sufficient data and pooled performance measures of these studies in a random effects meta-analysis (see Additional file 7). The pooled AUROC for predicting asthma exacerbations was 0.80 (95% CI 0.76–0.83), indicating a good discrimination ability (Fig. 3). The pooled sensitivity and specificity were 0.61 (95% CI 0.53–0.69, I2 = 98.71, P < 0.01) and 0.82 (95% CI 0.77–0.86, I2 = 99.95, P < 0.01), respectively (Fig. 4). Other values were as follows: PLR 3.33 (95% CI 2.73–4.07, I2 = 99.58, P < 0.01), NLR 0.47 (95% CI 0.39–0.57, I2 = 98.89, P < 0.01), and DOR 7.02 (95% CI 5.20–9.47, I2 = 100.00, P < 0.01) (see Additional file 8).
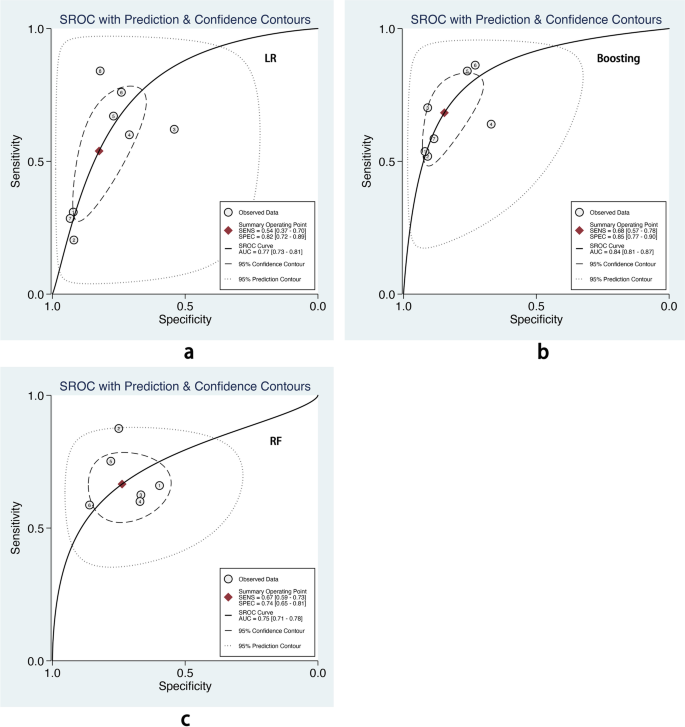
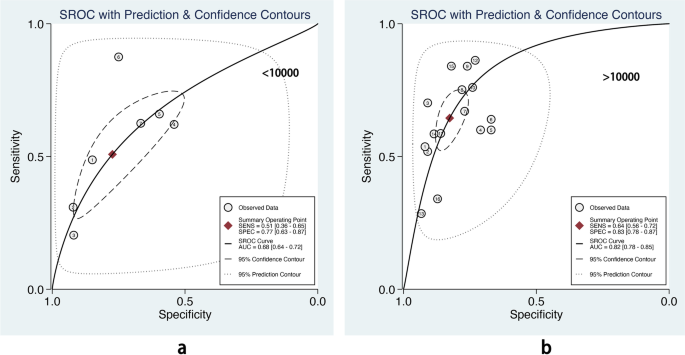
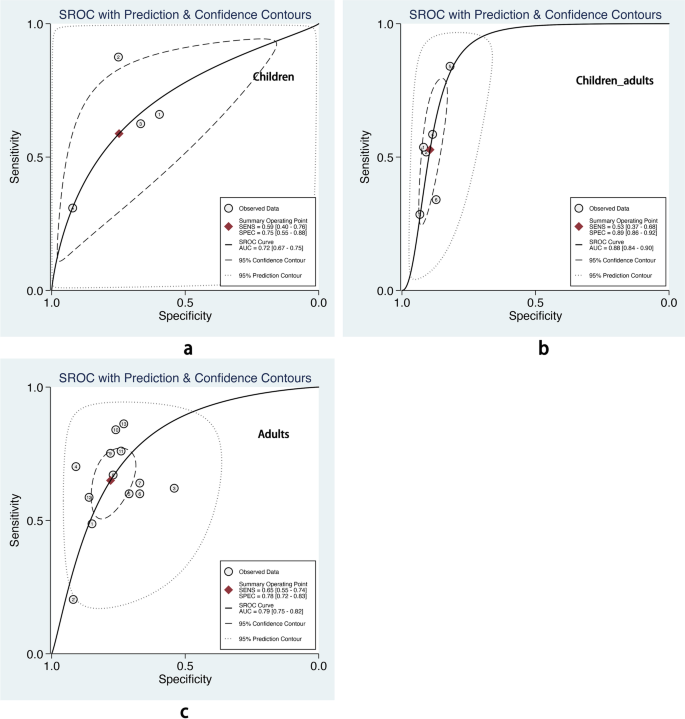
We also performed subgroup analysis stratified by ML methods, sample size, age group, and outcome definitions. As shown in Table 2, the overall pooled AUROC of boosting-based prediction models (0.84) was the highest, followed by studies using LR (0.77) and RF (0.75) (Table 2, Fig. 5). DOR, another measure of overall diagnostic ability, was also highest in studies applying boosting method (11.86). In studies with a large sample size (> 10000), the pooled AUROC and DOR were relatively high, with the number of 0.82 and 8.62, respectively (Table 2, Fig. 6). We classified outcome events as either emergency department visit/hospitalization (ED/HP) or in accordance with ATS/ERS statement (AE) definitions and performed subgroup analysis. The pooled AUROC in the two groups were similar, and the diagnostic odds ratio (DOR) was 7.58 for the ED/HP group and 6.01 for the AE group (Table 2, Fig. 7). Studies involving participants with children and adults had the highest pooled AUROC (0.88) and DOR (9.49) (Table 2, Fig. 8). Forest plots were shown in Additional file 9.
We perform the meta-regression analysis of the logit transformation of DOR due to the high level of heterogeneity. Univariate meta-regression analysis indicated that sample size and publication year contributed to the prediction power. However, only the coefficient of outcome definitions reached statistical significance in the multivariate model (Table 3). We included the outcome variable in the meta-regression analysis. The adjusted R-squared improved from 18.72% to 39.61%, and the Tau2 decreased from 0.4198 to 0.3118, indicating that the outcome variable could explain 25.7% heterogeneity.
Publication bias and sensitivity analysis
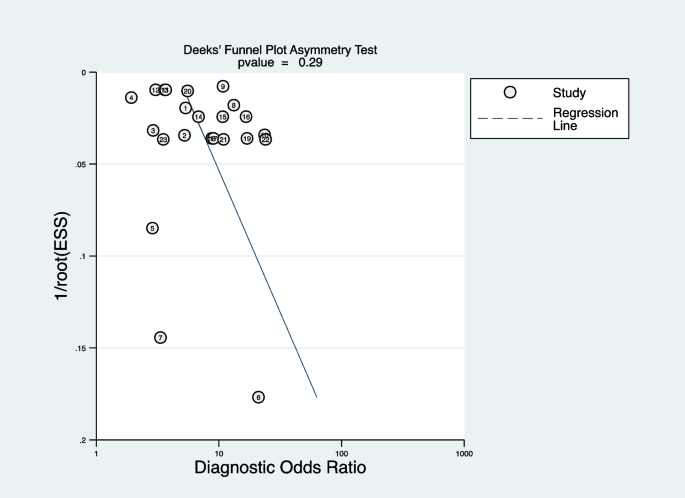
Deeks’ funnel plot was applied to test publication bias. As shown in Fig. 9, the funnel plot was symmetrical, indicating no publication bias (P = 0.29). Sensitivity analysis showed exclusion of any study did not affect the pooled estimations, suggesting the stability of the meta-analysis (see Additional file 10).
Discussion
Principal findings
This study systematically reviewed the ML-based prediction models for asthma exacerbations, which have not been discussed before. Eleven studies generated 23 ML prediction models, which were various in study design, data source, participants, outcome definitions, and ML algorithms. 6/11 studies were published in the recent three years, indicating a popular trend in applying ML algorithms in asthma. The overall pooled AUROC (0.8, 95% CI 0.76–0.83) and DOR (7.02, 95% CI 5.20–9.47) indicated that ML-based prediction models for asthma exacerbation could achieve good discrimination. ML prediction models could forecast patients at high risk of exacerbation from several days to years, helping identify patients needing closer management.
LR, boosting, and RF are the top three popular algorithms for asthma exacerbation prediction. According to the subgroup analysis, boosting-based prediction models had the highest pooled AUROC with a pooled AUROC of 0.84(95% CI 0.81–0.87), and the confidence interval of pooled AUROC was non-overlap with LR (0.77, 95% CI 0.73–0.81) and RF (0.75, 95% CI 0.71–0.78). Bridge et al. [8] conducted a systemic review and found that LR had a higher pooled c-statistic than optimal action points and CART in predicting asthma exacerbations. However, the authors did not include other ML methods. In this article, we found that boosting could also achieve good performance. It was potentially an alternative method in asthma exacerbation prediction, and more candidate models developed by ML should be tested.
The sample size is crucial for model performance. Compared with robust techniques like LR and CART, modern ML methods need higher times of events per variable to achieve stable performance [25]. Our subgroup analysis also showed that compared with prediction models with a smaller sample size (< 10000 participants), models developed in a big sample size (> 10000 participants) showed relatively high pooled AUROC (0.82, 95% CI 0.78–0.85 vs. 0.68, 95% CI 0.64–0.72) in the test dataset. This suggests that ML methods would be preferable for prediction models only if a large dataset is available [25].
As for predictors, the most important features were systemic steroids, short-acting beta2-agonists, age, ED visit, asthma diagnosis number, exacerbation history, race, BMI, duration, blood eosinophils, and smoking. Most of these factors were consistent with the risk factor identified in GINA (https://ginasthma.org/wp-content/uploads/2021/04/GINA-2021-Main-Report_FINAL_21_04_28-WMS.pdf) and previous studies [26, 27]. Other biomarkers, such as volatile organic compounds and single nucleotide polymorphisms were also used as input features to predict asthma exacerbations [19, 20]. However, these studies were performed with a small sample size of participants resulting in a high risk of overfitting. In addition, these factors require advanced equipment, limiting application in practice. Socioeconomic factors were included in only three studies but were identified as insignificant. Environmental factors, such as air pollutants, are also crucial for asthma exacerbation [28]. However, none of these studies focus on environmental factors.
Strengths and limitations
This study has several strengths. Firstly, we described included studies in detail and used logical methodology, which could provide a clear understanding of ML models in asthma exacerbation prediction. Additionally, the number of models allows us to conduct a meta-analysis of performance measures and compare different ML algorithms.
Despite the excellent prediction power of ML-based models confirmed in this study, several limitations are also identified. The main limitation was heterogeneity within studies. The difference in sample sizes, participants, feature selection, and prediction windows might affect the prediction ability of each model. Thus, the results analyzed in this study should be applied prudently. In addition, we did not include papers published in non-English, and we might not include all ML-based prediction models in the field of asthma exacerbations.
Future direction
ML methods are a potential way to achieve excellent performance in asthma exacerbation prediction, and more ML methods should be tested in the future. Although many models were developed, few of them were applied in practice. Therefore, improving the generalizability of prediction models in large separate datasets is crucial. Practicability is another critical factor. Simple models with a few predictors and using predictors that are easy to access could improve prediction models' practicability. Moreover, bundling ML algorithms to software or system would benefit in translating research into practice applications. Besides, randomized control studies are warranted to evaluate whether these models could benefit asthmatic patients by preventing asthma exacerbations.
Conclusion
Early identification of asthmatic patients at high risk of asthma exacerbations guides physicians to take closer management and timely intervention. This study showed that ML could achieve great performance in predicting asthma exacerbations. Future studies should focus on improving models' generalizability and practicability, thus driving the application of these models in clinical practice.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- ATS/ERS:
-
Official American thoracic society/European respiratory society
- CART:
-
Classification and regression trees
- DOR:
-
Diagnostic odds ratio
- GINA:
-
Global initiative for asthma
- LGBM:
-
Light gradient boosting machine
- LR:
-
Logistic regression
- ML:
-
Mchine learning
- NLR:
-
Negative likelihood ratio
- NN:
-
Neural network
- PLR:
-
Positive likelihood ratio
- PROBAST:
-
The prediction model risk of bias assessment tool
- RF:
-
Random forest
- ROB:
-
Risk of bias
- SVM:
-
Support vector machine
References
Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42:5–15.
Bergmann KC, Skowasch D, Timmermann H, Lindner R, Virchow JC, Schmidt O, et al. Prevalence of patients with uncontrolled asthma despite NVL/GINA Step 4/5 treatment in Germany. J Asthma Allergy. 2022;15:897–906.
Nagase H, Adachi M, Matsunaga K, Yoshida A, Okoba T, Hayashi N, et al. Prevalence, disease burden, and treatment reality of patients with severe, uncontrolled asthma in Japan. Allergol Int. 2020;69:53–60.
Loymans RJ, Ter Riet G, Sterk PJ. Definitions of asthma exacerbations. Curr Opin Allergy Clin Immunol. 2011;11:181–6.
Luskin AT, Chipps BE, Rasouliyan L, Miller DP, Haselkorn T, Dorenbaum A. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2:544-52.e1-2.
O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW, START Investigators Group. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. Epub 2008 Oct 31. Erratum in: Am J Respir Crit Care Med. 2010;182(7):983-984.
Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4:120-129.e3.
Bridge J, Blakey JD, Bonnett LJ. A systematic review of methodology used in the development of prediction models for future asthma exacerbation. BMC Med Res Methodol. 2020;20:22.
Beam AL, Kohane IS. Big data and machine learning in health care. JAMA. 2018;319:1317–8.
Tsang KCH, Pinnock H, Wilson AM, Shah SA. Application of machine learning algorithms for asthma management with mHealth: a clinical review. J Asthma Allergy. 2022;15:855–73.
Feng Y, Wang Y, Zeng C, Mao H. Artificial intelligence and machine learning in chronic airway diseases: focus on asthma and chronic obstructive pulmonary disease. Int J Med Sci. 2021;18:2871–89.
Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99.
Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. 2019;170:W1–33.
Luo G, Nau CL, Crawford WW, Schatz M, Zeiger RS, Rozema E, et al. Developing a predictive model for asthma-related hospital encounters in patients with asthma in a large, integrated health care system: secondary analysis. JMIR Med Inform. 2020;8:e22689.
Luo G, He S, Stone BL, Nkoy FL, Johnson MD. Developing a model to predict hospital encounters for asthma in asthmatic patients: secondary analysis. JMIR Med Inform. 2020;8:e16080.
Lieu TA, Capra AM, Quesenberry CP, Mendoza GR, Mazar M. Computer-based models to identify high-risk adults with asthma: is the glass half empty of half full? J Asthma. 1999;36:359–70.
Schatz M, Nakahiro R, Jones CH, Roth RM, Joshua A, Petitti D. Asthma population management: development and validation of a practical 3-level risk stratification scheme. Am J Manag Care. 2004;10:25–32.
Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Development and validation of a medication intensity scale derived from computerized pharmacy data that predicts emergency hospital utilization for persistent asthma. Am J Manag Care. 2006;12:478–84.
Xu M, Tantisira KG, Wu A, Litonjua AA, Chu JH, Himes BE, et al. Genome Wide Association Study to predict severe asthma exacerbations in children using random forests classifiers. BMC Med Genet. 2011;12:90.
van Vliet D, Smolinska A, Jöbsis Q, Rosias P, Muris J, Dallinga J, Dompeling E, van Schooten FJ. Can exhaled volatile organic compounds predict asthma exacerbations in children? J Breath Res. 2017;11:016016.
Tong Y, Messinger AI, Wilcox AB, Mooney SD, Davidson GH, Suri P, Luo G. Forecasting future asthma hospital encounters of patients with asthma in an academic health care system: predictive model development and secondary analysis study. J Med Internet Res. 2021;23:e22796.
Zein JG, Wu CP, Attaway AH, Zhang P, Nazha A. Novel machine learning can predict acute asthma exacerbation. Chest. 2021;159:1747–57.
Noble M, Burden A, Stirling S, Clark AB, Musgrave S, Alsallakh MA, et al. Predicting asthma-related crisis events using routine electronic healthcare data: a quantitative database analysis study. Br J Gen Pract. 2021;71:e948–57.
de Hond AAH, Kant IMJ, Honkoop PJ, Smith AD, Steyerberg EW, Sont JK. Machine learning did not beat logistic regression in time series prediction for severe asthma exacerbations. Sci Rep. 2022;12:20363.
van der Ploeg T, Austin PC, Steyerberg EW. Modern modeling techniques are data hungry: a simulation study for predicting dichotomous endpoints. BMC Med Res Methodol. 2014;14:137.
DiMango E, Rogers L, Reibman J, Gerald LB, Brown M, Sugar EA, et al. Risk factors for asthma exacerbation and treatment failure in adults and adolescents with well-controlled asthma during continuation and step-down therapy. Ann Am Thorac Soc. 2018;15:955–61.
McDowell PJ, Busby J, Hanratty CE, Djukanovic R, Woodcock A, Walker S, et al. Exacerbation profile and risk factors in a type-2-low enriched severe asthma cohort: a clinical trial to assess asthma exacerbation phenotypes. Am J Respir Crit Care Med. 2022;206:545–53.
Wang M, Li H, Huang S, Qian Y, Steenland K, Xie Y, Papatheodorou S, Shi L. Short-term exposure to nitrogen dioxide and mortality: a systematic review and meta-analysis. Environ Res. 2021;202:111766.
Acknowledgements
We would like to thank Yang Xie for obtaining the full text of included papers.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SQX drafted this manuscript; SQX, WC, and XYJ conducted the research search, duplication checking, and eligible studies selection. SQX and YJ performed the data extraction and statistical analysis. SQX and CHL performed the quality and bias assessment. CHL revised this manuscript. All authors approved the final version of this paper.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declared that they had no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Search term and results.
Additional file 2:
Methods.
Additional file 3:
Definitions of participants and outcomes of included studies.
Additional file 4:
The explanation of dataset split and validation methods.
Additional file 5:
Features and most important features in prediction models.
Additional file 6:
Risk of bias and applicability assessment based on PROBAST tools.
Additional file 7:
11 studies included in the meta-analysis.
Additional file 8:
The overall pooled positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio of 23 machine learning prediction models.
Additional file 9:
Forest plots of performance measures in subgroup analysis.
Additional file 10:
The influence of each model for the outcome of meta-analysis
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xiong, S., Chen, W., Jia, X. et al. Machine learning for prediction of asthma exacerbations among asthmatic patients: a systematic review and meta-analysis. BMC Pulm Med 23, 278 (2023). https://doi.org/10.1186/s12890-023-02570-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02570-w