- Research
- Open access
- Published:
The association between ibuprofen administration in children and the risk of developing or exacerbating asthma: a systematic review and meta-analysis
BMC Pulmonary Medicine volume 24, Article number: 412 (2024)
Abstract
Background
Ibuprofen is one of the most commonly used analgesic and antipyretic drugs in children. However, its potential causal role in childhood asthma pathogenesis remains uncertain. In this systematic review, we assessed the association between ibuprofen administration in children and the risk of developing or exacerbating asthma.
Methods
We searched MEDLINE, Embase, Cochrane Library, CINAHL, Web of Science, and Scopus from inception to May 2022, with no language limits; searched relevant reviews; and performed citation searching. We included studies of any design that were primary empirical peer-reviewed publications, where ibuprofen use in children 0–18 years was reported. Screening was performed in duplicate by blinded review. In total, 24 studies met our criteria. Data were extracted according to PRISMA guidelines, and the risk of bias was assessed using RoB2 and NOS tools. Quantitative data were pooled using fixed effect models, and qualitative data were pooled using narrative synthesis. Primary outcomes were asthma or asthma-like symptoms. The results were grouped according to population (general, asthmatic, and ibuprofen-hypersensitive), comparator type (active and non-active) and follow-up duration (short- and long-term).
Results
Comparing ibuprofen with active comparators, there was no evidence of a higher risk associated with ibuprofen over both the short and long term in either the general or asthmatic population. Comparing ibuprofen use with no active alternative over a short-term follow-up, ibuprofen may provide protection against asthma-like symptoms in the general population when used to ease symptoms of fever or bronchiolitis. In contrast, it may cause asthma exacerbation for those with pre-existing asthma. However, in both populations, there were no clear long-term follow-up effects.
Conclusions
Ibuprofen use in children had no elevated risk relative to active comparators. However, use in children with asthma may lead to asthma exacerbation. The results are driven by a very small number of influential studies, and research in several key clinical contexts is limited to single studies. Both clinical trials and observational studies are needed to understand the potential role of ibuprofen in childhood asthma pathogenesis.
Background
Asthma is a noncommunicable disease affecting approximately 235 million people worldwide and is characterised by inflammation and narrowing of the small airways in the lungs, leading to any combination of cough, wheeze, shortness of breath, and chest tightness [1]. The prevalence of asthma has increased in many countries in recent decades, especially among children, making asthma a serious global public health problem [2, 3]. The reason for increasing asthma prevalence in children is uncertain, but there is likely a complex interaction of multiple risk factors, including environmental (e.g., increased air pollution, changes to housing conditions) and lifestyle factors (e.g., decreased physical activity, changes in diet, increased childhood obesity) [4].
Increased early-life use of pharmacological agents, such as analgesics and antipyretics, could be causal factors in childhood asthma pathogenesis. Due to fears of a causal relationship between aspirin use and Reye’s syndrome [5] and the risk of aspirin-induced asthma [6], aspirin use in children has dramatically decreased in recent decades. Consequently, drugs such as ibuprofen and paracetamol have become increasingly popular for treating fever and pain in children. In the United Kingdom, the National Health Service describes both paracetamol and ibuprofen as safe for treating pain and high temperature in babies and children [7]. However, caution is advised for ibuprofen use in children with asthma [8], while no such warning is supplied for paracetamol [9], suggesting that ibuprofen may be linked to asthma development or exacerbation in those with pre-existing asthma.
Ibuprofen is a non-steroidal anti-inflammatory drug (NSAID) that is frequently prescribed or administered over-the-counter (OTC) to treat fever and pain. Links between childhood ibuprofen use and asthma development or exacerbation are being investigated [10,11,12,13,14,15,16]. Ibuprofen’s inhibition of the cyclooxygenase system can lead to activation of the lipoxygenase system, resulting in bronchospasm [6, 17], which could precipitate asthma. Additionally, empirical evidence exists demonstrating ibuprofen-induced asthma exacerbation in children with asthma and self-reported aspirin allergy [18].
Despite these points, two recent systematic reviews did not identify a risk difference between ibuprofen and paracetamol in asthma development or exacerbation in children [14, 16]. However, one of these reviews limited the scope to randomised controlled trials (RCTs) [14], and the other to a relatively narrow age range of less than 2 years [16], restricting the generalisability of the findings.
We conducted a systematic review to assess the association between ibuprofen administration in children and the risk of developing or exacerbating asthma. The aim was to expand on previous reviews by looking across the entire age range of childhood from 0 to 18 years, including both interventional and observational studies, and assessing the association separately for clinically distinct paediatric subpopulations: general, asthmatic, and ibuprofen-hypersensitive.
Methods
Protocol development
We registered our review on PROSPERO on 8 July 2022 (CRD42022344838). The protocol was written according to PRISMA-P guidelines [19, 20] and made publicly available on OSF prior to registration with PROSPERO. Further methodological details can be found in our online protocol (https://doi.org/10.17605/OSF.IO/Z37KW).
Eligibility criteria
A full list of eligibility criteria is provided in Supplementary Methods S1.1 (Supplementary Tables 1–2). The numeric results from studies included in our review were grouped by population for synthesis: (i) general population of children (i.e., studies not limiting eligibility to specific clinical subpopulations; however, some study-specific exclusion will always occur, for example, children with severe asthma, ibuprofen hypersensitivity, or other contraindications for safety reasons; children with conditions that could interfere with ibuprofen administration or absorption, such as inability to swallow or frequent vomiting; children receiving treatments that could interfere with the outcome assessment, such as leukotriene receptor antagonist and other anti-asthmatic treatments); (ii) children with asthma; and (iii) children with ibuprofen hypersensitivity.
Search strategy
We searched six bibliographic databases (MEDLINE, Embase, Cochrane Library, CINAHL, Web Of Science, Scopus) to identify records on 21-May-2022, and our searches were independently peer-reviewed using the PRESS Checklist [21, 22] by an outreach librarian at the Bodleian Health Care Libraries, University of Oxford (https://doi.org/10.17605/OSF.IO/R3AV6). All search strategies are provided in full in Supplementary Methods S1.2. Additional information sources included relevant reviews that were identified during screening [10,11,12,13,14,15,16] and backwards citation searching using the citationchaser tool [23]. EPPI-Reviewer [24] was used for de-duplication, and screening was performed independently in duplicate, with disagreements settled by discussion between both reviewers.
Data extraction and bias assessment
Data extraction and bias assessment were performed by one reviewer and then verified by a second reviewer, with disagreements settled by discussion. Our primary outcomes of interest were asthma, asthma-like symptoms, or asthma exacerbation [2]. For risk of bias assessment, the Cochrane risk of bias tool (RoB2) was used for RCTs [25], and the Newcastle-Ottawa Scale (NOS) [26] was used for observational studies. The results from these assessments were used to decide which studies to include in primary syntheses (Supplementary Figs. 1–2). Our approach to assessing meta-biases (outcome reporting and publication biases) is detailed in Supplementary Methods S1.3.
Data synthesis
A narrative synthesis was performed when outcomes were too heterogeneous to synthesise quantitatively. Otherwise, meta-analysis was performed using the R package meta [27]. Given the sparsity of the data for quantitative synthesis, we report the common effect model results as primary results. For completion, we report additional analysis outputs, e.g., both odds and risk ratios; both common and random effects model effect sizes; I2, tau2, and chi2 for heterogeneity. Due to the sparsity of the results, subgroup analyses were not performed.
For meta-analysis of dichotomous data, ORs were pooled using Peto’s method [28] due to zero events in some arms. Where multiple outcomes from a study were available, the primary analysis was performed by selecting the outcomes with the expected lowest risk of bias. To test the robustness of the primary analysis, sensitivity analyses were performed using alternative combinations of studies’ numeric results.
Results
Study selection characteristics
Of the 820 records screened, 18 relevant studies were identified, with a further 6 from relevant reviews (Supplementary Fig. 3). The study characteristics for all 24 studies are summarised in Table 1. Relevant numeric results were grouped by population: (i) general population of children (Table 2), (ii) children with asthma (Table 3), and (iii) children with ibuprofen hypersensitivity (Table 4). For the general population and children with asthma, data synthesis was performed for (i) ibuprofen versus an active comparator (Fig. 1) and (ii) ibuprofen versus baseline (i.e., children not taking an alternative antipyretic or analgesic). To increase homogeneity, the results were also grouped based on the duration of follow-up, in line with a recent similar systematic review [16]: short duration of ≤ 28 days or long duration of > 28 days.
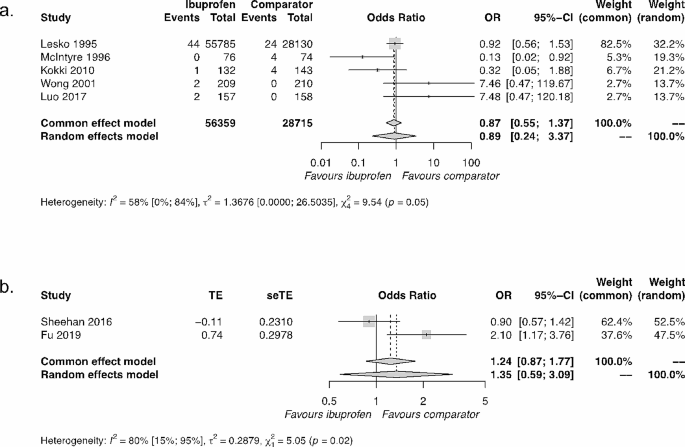
Synthesis of results of ibuprofen versus active comparators. The active comparator for Kokki 2010 was ketoprofen; for all other studies, the active comparator was paracetamol. (a) General population of children over a short duration. (b) Children with asthma over a long duration. Abbreviations: OR = odds ratio; 95% CI = 95% confidence interval
General population
In total, 13 numeric results from 9 studies relevant to assessing ibuprofen use in a general population of children were identified [29,30,31,32,33,34,35,36,37] (Table 2).
Ibuprofen versus active comparator
There were six results from six interventional studies (all RCTs) and two results from one observational cohort study that compared ibuprofen use with an active comparator in the general population. The main active comparator was paracetamol, with one study [29] using ketoprofen (Table 2). The durations of study for the interventional RCT were all short (≤ 28 days). Two of these results were from publications based on the same dataset, the Boston University Fever Study [30, 31], of which the original publication was selected for primary analysis.
The synthesis of five results comparing ibuprofen with active comparators (four paracetamol, one ketoprofen) resulted in a common effect OR = 0.87; 95% CI=[0.55, 1.37], demonstrating a lack of significant difference between ibuprofen and active comparators (Fig. 1a). Our sensitivity analyses were in agreement with this primary result (Supplementary Fig. 4).
A single observational study [36] assessed ibuprofen relative to paracetamol over both short and long durations (Table 2) in a general population of children. Over a short duration (14 days), no significant difference in wheezing was identified, but over a long duration (1 year), they observed a significant advantage to ibuprofen over paracetamol, with a reduction in health care practitioner visits for wheezing illness consistent with bronchiolitis or asthma.
Taken together, these interventional and observational results suggest that there is no difference between ibuprofen and active comparators in the general population over a short duration (≤ 28 days). This finding is driven largely by a single study, the Boston University Fever Study [31], conducted almost 30 years ago on a large sample (n = 83,915) of children aged 6 months to 12 years. Over longer follow-up durations of one year, there is evidence from only a single cohort study [36] to suggest that there may be a reduction in wheezing when ibuprofen is prescribed, rather than paracetamol, for a first episode of bronchiolitis in children aged 0–12 months.
Ibuprofen versus baseline
Five numeric results from three studies relevant to assessing ibuprofen relative to baseline (children not taking an alternative antipyretic or analgesic) in the general population were identified (Table 2). All outcomes were from observational studies. Due to the sparsity and substantive heterogeneity of the results, quantitative synthesis was not possible.
Two studies looked at general populations over short durations (≤ 28 days) [33, 36]. Both studies suggest that ibuprofen might decrease wheezing when taken for either acute febrile illness or bronchiolitis (Table 2).
Two studies looked at general populations of children over long durations [35, 36] and produced conflicting results. One study [36] compared those prescribed ibuprofen for a first episode of bronchiolitis to those not prescribed ibuprofen (or another drug) and followed up participants over a 1-year duration, observing a positive impact of ibuprofen prescription. The second study [35] compared children administered ibuprofen to those not administered ibuprofen during the first postnatal year and followed-up participants at a 3–5 year duration, observing a negative impact of ibuprofen on asthma development, and at a 7–10 year duration, observing no difference between cohorts (Table 2).
Taken together, ibuprofen use in the general population of children during acute febrile illness or bronchiolitis might decrease wheezing when assessed in the short-term (≤ 28 days), with both observational studies reporting strong significant effects (Table 2). Over longer durations, the two observational studies identified in this review have substantive heterogeneity in design, analysis, and outcome, preventing meaningful synthesis. Additionally, their numeric findings are inconsistent (Table 2).
Asthmatic population
Five numeric results from four studies relevant to assessing ibuprofen in asthmatic paediatric populations were identified [38,39,40,41] (Table 3).
Ibuprofen versus active comparator
Three results across three studies compared ibuprofen with an active comparator (paracetamol in all cases) in asthmatic populations (Table 3). One interventional study assessed outcomes over a short duration [39] and found no difference between treatments. While further analyses in this paper did suggest a favourable outcome for ibuprofen relative to paracetamol, the results from this second post-hoc Boston Fever Study report are at very high risk of bias in the selection of the reported result (Supplementary Fig. 2).
Two studies looked at the comparison between ibuprofen and paracetamol in asthmatic populations over long durations [38, 41]. The RCT study [41] identified no difference between drugs (OR = 0.90 [ 0.57, 1.41]). In contrast, the observational cohort study [38] identified a significant disadvantage for ibuprofen relative to paracetamol in asthmatic populations (aOR = 2.10 [1.17, 3.76]). These conflicting results for ibuprofen relative to paracetamol in asthmatic populations over long durations are challenging to resolve due to the different experimental designs. However, there are also several similarities in their designs: use of the same active comparator, inclusion of asthmatic populations of children with similar age ranges (Sheehan: 1–4.9 years; Fu: 1–5 years) over similar follow-up durations (Sheehan: 46 weeks; Fu: 52 weeks), and use of asthma exacerbation as the outcome. As an exploratory analysis, we synthesised these results, which resulted in a common effect OR = 1.24; 95% CI=[0.87, 1.77], suggesting an overall non-significant effect, which is consistent with the RCT study result alone (Fig. 1b).
Taken together, these interventional and observational results suggest that there is no difference in asthma exacerbation between ibuprofen and paracetamol in asthmatic populations over short or long durations.
Ibuprofen versus baseline
Only a single study looked at an asthmatic population over both short and long durations [40]. Over a short duration, this study found that ibuprofen increased asthma exacerbation. Over a long duration, they found no effect of ibuprofen on asthma exacerbation in the asthmatic population.
Ibuprofen hypersensitive population
Four drug provocation studies were identified that studied ibuprofen-hypersensitive children where ibuprofen was ingested and adverse events reported as part of hypersensitivity diagnosis [42,43,44,45]. A range of respiratory adverse effects were reported that included asthma, coughing, wheezing, dyspnoea, and respiratory distress (Table 4). Across the four studies, there was a total of 10 children with respiratory adverse events reported in a total of 80 children. Thus, in children with ibuprofen hypersensitivity, the average rate of respiratory adverse events following ibuprofen ingestion was 12.5%.
Unsynthesised papers
Seven studies were identified that reported the relationship between ibuprofen and asthma in children, which were not synthesised in this review [18, 46,47,48,49,50,51]: five studies reported on single cases, and two group analysis studies had substantive differences in methodology and outcomes relative to other studies included in this review.
One crossover RCT [46] assessed the prevalence of ibuprofen-sensitive asthma in children with mild or moderate persistent asthma using bronchoprovocation challenge and found a prevalence of 2%. Another non-randomised controlled study [18] assessed the impact of short-term ibuprofen treatment on pulmonary function in children with mild to moderate stable asthma and self-reported aspirin allergy. Relative to a healthy control group, the asthmatic group exhibited a drop in FEV1 (forced expiratory volume in the first second) of 18.85% and an increase in FeNO (fractional exhaled nitric oxide) of 20.76 ppb. A summary of the results from these two studies is provided in Supplementary Table 3.
Four case reports of severe adverse events to ibuprofen were identified [47, 48, 50, 51], and in all cases, the children had pre-existing asthma. Last, in a case series of fatal asthma in Finland, a single death due to ibuprofen ingestion was reported in a child with severe asthma and a known allergy to ibuprofen [49].
Discussion
Here, we assessed the association between ibuprofen use and asthma in children aged 0–18 years. Both observational and interventional studies were reviewed in the general population as well as the asthmatic population. Studies that benchmarked ibuprofen against an active comparator almost exclusively used paracetamol, and in both populations of children, the combined evidence suggested no difference in asthma-related adverse events between ibuprofen and paracetamol (or ketoprofen) use. A single observational study suggested a potential benefit of ibuprofen over paracetamol prescription in response to bronchiolitis in the general paediatric population after a one-year follow-up. When ibuprofen use was assessed relative to no alternative drug administration, differences emerged between the general and asthmatic populations. In the short-term follow-up (1–14 days) to ibuprofen use, two observational studies reported favourable effects in the general population, while one observational and one interventional study observed unfavourable effects in the asthmatic population. Over a longer follow-up period (12 weeks to 10 years), no clear effect emerged for either population.
The majority of research on the association between ibuprofen use and asthma-related adverse events in children has been conducted in the general population, benchmarked relative to paracetamol, and participants followed-up over a short duration [29,30,31,32, 34, 36, 37]. The aggregate result from five RCTs conducted in this context is driven primarily by the Boston University Fever Study [31], conducted almost 30 years ago on children aged 6 months to 12 years. While a single observational study [36] conducted five years ago corroborates this finding, research is sparse. Furthermore, only a single study comparing ibuprofen with paracetamol use with a short-term follow-up was conducted in children with asthma [39], and this study was a second post-hoc analysis publication of the same Boston University Fever Study dataset. Given the increased vulnerability of the asthmatic population to respiratory adverse events from ibuprofen use that was observed in our review, there is a clear lack of research comparing the short-term effects of ibuprofen relative to alternative analgesics and antipyretics such as paracetamol in children with asthma.
Two studies [38, 41] assessing differences between ibuprofen and paracetamol use over longer follow-up periods in asthmatic populations report conflicting results. Due to several study similarities, we tentatively synthesised the two results, and no aggregate difference between ibuprofen and paracetamol was observed. However, in the RCT [41], the median dose of trial medication (ibuprofen or paracetamol) was 5.5 doses (IQR = 1–15) and matched between trial arms. In the retrospective cohort study [38], it could not be determined by the original investigators whether patients took the medication prescribed. Additionally, the observational study did not control for upper respiratory tract infections, a well-documented source of confounding by indication [35, 52], which were not well-matched between the ibuprofen and paracetamol cohorts. For these reasons, the RCT finding alone or the synthesised outcome of no difference between drugs seems most justifiable.
Comparing the asthmatic and general populations for short-term asthma-relevant outcomes after ibuprofen use, no conflicts in results were observed. The two observational studies in the general population [33, 36] both observed reductions in asthma-related outcomes, while one observational [40] and one interventional [18] study in the asthmatic population both observed increases in asthma-related outcomes. These findings highlight the importance of avoiding naïve pooling of results from studies in these different paediatric populations.
It is noteworthy that all RCTs reviewed compared ibuprofen with an active comparator. Of the studies comparing ibuprofen with a baseline of no alternative drug, three were cohort studies [35, 36, 40], and one was cross-sectional [33]. One non-randomised interventional study [18] compared an asthmatic sample with a healthy control sample. This highlights one of the limitations of the RCT design approach in assessing adverse events in the youngest children [53, 54]. As a recent RCT feasibility study found [55], almost three quarters of parents surveyed described the use of a placebo comparator treatment as unacceptable for treating their child’s fever or pain. This ethical unacceptability of using a placebo arm in clinical trials for treating pain and fever in young children [55, 56] introduces an ambiguity into these active comparator RCT studies, as a lack of difference among active comparators does not exclude the possibility that both ibuprofen and active comparator use may be associated with parallel increases in asthma exacerbations [41, 56]. It has been argued that, given that ibuprofen and paracetamol have different mechanisms of action, it is unlikely that their use could be associated with similar increases in the rate of asthma-related complications that are known to be determined by disparate mechanisms of disease [41, 56]. However, this speculation requires careful examination and empirical support. Observational studies with comparator groups in which an active treatment was not prescribed or taken can be used as a baseline control to assess the impact of ibuprofen alone, acknowledging the challenges of inferring causality in observational studies. It is these advantages and disadvantages of both RCTs and observational designs that require a review of the association between ibuprofen use and asthma-related outcomes in children to consider and attempt to synthesise all study design types. This feature of our review adds substantially to two recent systematic reviews in this area [14, 56] that either limited the study designs to RCTs [14] or limited the population to those under 2 years [56].
We identified four drug provocation trials in which ibuprofen hypersensitivity was confirmed in children by controlled administration of ibuprofen [42,43,44,45] and respiratory adverse events were recorded. The average percentage of children with confirmed ibuprofen hypersensitivity who displayed respiratory adverse events was 12.5%. Relative to other adverse events, such as angio-oedema and urticaria (which were by far the most common adverse events), asthma and asthma-like respiratory events were less commonly reported. While adverse respiratory reactions to ibuprofen ingestion in those with ibuprofen hypersensitivity can be quite severe, as reported in a handful of case reports [47, 48, 50, 51], fatalities appear to be very rare. In this review, only a single case of ibuprofen-induced asthma fatality was identified [49].
The number of studies in this review that were relevant to important clinical populations and contexts was unfortunately sparse. Only a single publication was identified for each of the following three contexts: the general population where ibuprofen is compared with an active comparator with a follow-up duration longer than 1 month [36]; the asthmatic population where ibuprofen is compared with an active comparator with a short-term follow-up [39]; and the asthmatic population where ibuprofen is compared with a baseline of no active comparator with a follow-up duration longer than 1 month [40]. These limitations hinder the generalisability of findings to several important clinical contexts and are an ongoing issue to be addressed.
Conclusion
Here, we found that research is most lacking for populations of children with pre-existing asthma, who are the population at most risk for potential respiratory adverse events following ibuprofen use. Our review highlights the importance of assessing both interventional and observational studies and analysing the general population and asthmatic population separately. Continued investigation into the role of early-life ibuprofen use and its short-term and long-term impact on childhood asthma is needed.
Data availability
All data (data collection form, risk of bias assessment forms, and data used for all analyses) are publicly available on the project’s OSF site: https://doi.org/10.17605/OSF.IO/ZBDS7. All code used for the meta-analysis is publicly available on Zenodo: https://doi.org/10.5281/zenodo.11258287.
References
WHO. Asthma [Internet]. 2023 [cited 2023 Apr 4]. https://www.who.int/news-room/fact-sheets/detail/asthma
Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. European Respiratory Journal [Internet]. 2022 Jan 1 [cited 2022 Apr 27];59(1). https://erj.ersjournals.com/content/59/1/2102730
Vos T, Murray CJL, GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59.
Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. 2015;386(9998):1075–85.
Schrör K. Aspirin and Reye syndrome: a review of the evidence. Paediatr Drugs. 2007;9(3):195–204.
Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis and management. J Allergy Clin Immunol. 1999;104(1):5–13.
NHS. nhs.uk. 2020 [cited 2023 Apr 4]. Medicines for babies and children. https://www.nhs.uk/conditions/baby/health/medicines-for-babies-and-children/
NHS. nhs.uk. 2022 [cited 2023 Apr 4]. Who can and cannot take ibuprofen for children. https://www.nhs.uk/medicines/ibuprofen-for-children/who-can-and-cannot-take-ibuprofen-for-children/
NHS. nhs.uk. 2022 [cited 2023 Apr 4]. Who can and cannot take paracetamol for children. https://www.nhs.uk/medicines/paracetamol-for-children/who-can-and-cannot-take-paracetamol-for-children/
Kanabar D, Dale S, Rawat M. A review of ibuprofen and acetaminophen use in febrile children and the occurrence of asthma-related symptoms. Clin Ther. 2007;29(12):2716–23.
Kanabar DJ. A clinical and safety review of Paracetamol and Ibuprofen in children. Inflammopharmacology. 2017;25(1):1–9.
Kauffman RE, Lieh-Lai M. Ibuprofen and increased morbidity in children with asthma: fact or fiction? Paediatr Drugs. 2004;6(5):267–72.
Pierce CA, Voss B. Efficacy and safety of ibuprofen and acetaminophen in children and adults: a meta-analysis and qualitative review. Ann Pharmacother. 2010;44(3):489–506.
Sherbash M, Furuya-Kanamori L, Nader JD, Thalib L. Risk of wheezing and asthma exacerbation in children treated with Paracetamol versus Ibuprofen: a systematic review and meta-analysis of randomised controlled trials. BMC Pulm Med. 2020;20(1):72.
Southey ER, Soares-Weiser K, Kleijnen J. Systematic review and meta-analysis of the clinical safety and tolerability of ibuprofen compared with paracetamol in paediatric pain and fever. Current medical research and opinion [Internet]. 2009 Sep [cited 2022 May 19];25(9). https://pubmed.ncbi.nlm.nih.gov/19606950/
Tan E, Braithwaite I, McKinlay CJD, Dalziel SR. Comparison of Acetaminophen (paracetamol) with Ibuprofen for Treatment of Fever or Pain in children younger than 2 years: a systematic review and Meta-analysis. JAMA Netw Open. 2020;3(10):e2022398.
Szczeklik A. The cyclooxygenase theory of aspirin-induced asthma. Eur Respir J. 1990;3(5):588–93.
Su YM, Huang CS, Wan KS. Short-term ibuprofen treatment and pulmonary function in children with asthma. Indian Pediatr. 2015;52(8):691–3.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Reviews. 2015;4(1):1.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Lefebvre C, PRESS Peer Review of Electronic Search Strategies. : 2015 Guideline Explanation and Elaboration (PRESS E&E). CADTH Methods and Guidelines [Internet]. 2016 [cited 2022 Mar 3]; https://www.cadth.ca/press-peer-review-electronic-search-strategies-2015-guideline-explanation-and-elaboration
Haddaway NR, Grainger MJ, Gray CT. citationchaser: An R package and Shiny app for forward and backward citations chasing in academic searching [Internet]. Zenodo; 2021 [cited 2023 Jan 17]. https://zenodo.org/record/4543513
Thomas J, Graziosi S, Brunton J, Ghouze Z, O’Driscoll P, Bond M et al. EPPI-Reviewer: advanced software for systematic reviews, maps and evidence synthesis [Internet]. EPPI-Centre, UCL Social Research Institute, University College London; 2022. http://eppi.ioe.ac.uk/cms/Default.aspx?tabid=2914
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. 2008 [cited 2022 May 23]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60.
Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335–71.
Kokki H, Kokki M. Ketoprofen versus Paracetamol (acetaminophen) or ibuprofen in the management of fever: results of two randomized, double-blind, double-dummy, parallel-group, repeated-dose, multicentre, phase III studies in children. Clin Drug Investig. 2010;30(6):375–86.
Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics. 1999;104(4):e39.
Lesko SM, Mitchell AA. An assessment of the safety of pediatric ibuprofen. A practitioner-based randomized clinical trial. JAMA. 1995;273(12):929–33.
Luo S, Ran M, Luo Q, Shu M, Guo Q, Zhu Y, et al. Alternating Acetaminophen and Ibuprofen versus monotherapies in improvements of distress and reducing refractory fever in Febrile children: a Randomized Controlled Trial. Paediatr Drugs. 2017;19(5):479–86.
Matok I, Elizur A, Perlman A, Ganor S, Levine H, Kozer E. Association of Acetaminophen and Ibuprofen Use with Wheezing in Children with Acute Febrile illness. Ann Pharmacother. 2017;51(3):239–44.
McIntyre J, Hull D. Comparing efficacy and tolerability of Ibuprofen and Paracetamol in fever. Arch Dis Child. 1996;74(2):164–7.
Sordillo JE, Scirica CV, Rifas-Shiman SL, Gillman MW, Bunyavanich S, Camargo CA, et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol. 2015;135(2):441–8.
Walsh P, Rothenberg SJ. Wheezing after the use of acetaminophen and or ibuprofen for first episode of bronchiolitis or respiratory tract infection. PLoS ONE. 2018;13(9):e0203770.
Wong A, Sibbald A, Ferrero F, Plager M, Santolaya ME, Escobar AM, et al. Antipyretic effects of dipyrone versus ibuprofen versus acetaminophen in children: results of a multinational, randomized, modified double-blind study. Clin Pediatr (Phila). 2001;40(6):313–24.
Fu LS, Lin CC, Wei CY, Lin CH, Huang YC. Risk of acute exacerbation between acetaminophen and ibuprofen in children with asthma. PeerJ. 2019;7:e6760.
Lesko SM, Louik C, Vezina RM, Mitchell AA. Asthma morbidity after the short-term use of ibuprofen in children. Pediatrics. 2002;109(2):E20.
Lo PC, Tsai YT, Lin SK, Lai JN. Risk of asthma exacerbation associated with nonsteroidal anti-inflammatory drugs in childhood asthma: a nationwide population-based cohort study in Taiwan. Med (Baltim). 2016;95(41):e5109.
Sheehan WJ, Mauger DT, Paul IM, Moy JN, Boehmer SJ, Szefler SJ, et al. Acetaminophen versus Ibuprofen in Young children with mild persistent asthma. N Engl J Med. 2016;375(7):619–30.
Corzo JL, Zambonino MA, Muñoz C, Mayorga C, Requena G, Urda A, et al. Tolerance to COX-2 inhibitors in children with hypersensitivity to nonsteroidal anti-inflammatory drugs. Br J Dermatol. 2014;170(3):725–9.
Ertoy Karagol HI, Yilmaz O, Topal E, Ceylan A, Bakirtas A. Nonsteroidal anti-inflammatory drugs-exacerbated respiratory disease in adolescents. Int Forum Allergy Rhinol. 2015;5(5):392–8.
Guvenir H, Dibek Misirlioglu E, Vezir E, Toyran M, Ginis T, Civelek E et al. Nonsteroidal anti-inflammatory drug hypersensitivity among children. Allergy Asthma Proc. 2015;36(5):386–93.
Yilmaz Topal O, Kulhas Celik I, Turgay Yagmur I, Toyran M, Civelek E, Karaatmaca B, et al. Results of NSAID provocation tests and difficulties in the classification of children with nonsteroidal anti-inflammatory drug hypersensitivity. Ann Allergy Asthma Immunol. 2020;125(2):202–7.
Debley JS, Carter ER, Gibson RL, Rosenfeld M, Redding GJ. The prevalence of ibuprofen-sensitive asthma in children: a randomized controlled bronchoprovocation challenge study. J Pediatr. 2005;147(2):233–8.
Goraya JS, Virdi VS. To the editor: exacerbation of asthma by ibuprofen in a very young child. Pediatr Pulmonol. 2001;32(3):262.
King G, Byrne A, Fleming P. A case of severe NSAID exacerbated respiratory disease (NERD) following a dental procedure in a child. Eur Arch Paediatr Dent. 2016;17(4):277–81.
Malmström K, Kaila M, Kajosaari M, Syvänen P, Juntunen-Backman K. Fatal asthma in Finnish children and adolescents 1976–1998: validity of death certificates and a clinical description. Pediatr Pulmonol. 2007;42(3):210–5.
Menendez R, Venzor J, Ortiz G. Failure of zafirlukast to prevent ibuprofen-induced anaphylaxis. Ann Allergy Asthma Immunol. 1998;80(3):225–6.
Palmer GM. A teenager with severe asthma exacerbation following ibuprofen. Anaesth Intensive Care. 2005;33(2):261–5.
Schnabel E, Heinrich J. Respiratory tract infections and not Paracetamol medication during infancy are associated with asthma development in childhood. J Allergy Clin Immunol. 2010;126(5):1071–3.
CRD. Chapter 4: systematic reviews of adverse effects. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York Publishing Services; 2009.
Reeves B, Deeks J, Higgins J, Shea B, Tugwell P, Wells G. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, editors. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. 6.3. Cochrane; 2022 [cited 2023 Apr 7]. https://training.cochrane.org/handbook/current/chapter-24
Riley J, Braithwaite I, Shirtcliffe P, Caswell-Smith R, Hunt A, Bowden V, et al. Randomized controlled trial of asthma risk with Paracetamol use in infancy–a feasibility study. Clin Exp Allergy. 2015;45(2):448–56.
Tan E, Braithwaite I, McKinlay C, Riley J, Hoare K, Okesene-Gafa K, et al. Randomised controlled trial of Paracetamol or Ibuprofen, as required for fever and pain in the first year of life, for prevention of asthma at age 6 years: Paracetamol or Ibuprofen in the primary prevention of asthma in Tamariki (PIPPA Tamariki) protocol. BMJ Open. 2020;10(12):e038296.
Acknowledgements
We thank Imran Lodhi, Fiona Murray-Zmijewski, Frederic Esclassan, and Bill Laughey for reviewing and advising on improvements for this systematic review. We thank Carolyn Smith, the Outreach Librarian at University of Oxford’s Bodleian Libraries, who performed the search strategy PRESS Peer Review.
Funding
This work was funded by Reckitt. Employees of Reckitt were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of a manuscript; and decision regarding where to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
LB: conceptualization, methodology, data curation, formal analysis, investigation, visualization, writing – original draft, writing – review & editing. MC: methodology, data curation, investigation, validation, writing – review & editing. AB: conceptualization, methodology, data curation, investigation, writing – review & editing. RS: conceptualization, methodology, data curation, investigation, writing – review & editing. OS: conceptualization, methodology, project administration, writing – review & editing. NS: conceptualization, methodology, project administration, writing – review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
OS and NS are current employees of Reckitt and may hold equity interest in Reckitt. LB and RS were compensated by Reckitt for activities related to execution of the study. MC and AB declare no competing interests. No other disclosures were reported.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baxter, L., Cobo, M.M., Bhatt, A. et al. The association between ibuprofen administration in children and the risk of developing or exacerbating asthma: a systematic review and meta-analysis. BMC Pulm Med 24, 412 (2024). https://doi.org/10.1186/s12890-024-03179-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03179-3